Experts Answer Parents’ Questions About COVID-19 Vaccine for Kids Ages 5-11
UC Davis Health physician, epidemiologist offer insights following FDA and CDC expanded vaccine authorization
(SACRAMENTO) Now that the Food and Drug Administration (FDA) and Centers for Disease Control (CDC) have authorized the COVID-19 vaccine for children ages 5-11, parents may have questions about vaccinating their children.
COVID-19 has taken the greatest toll on the elderly, but since the release of the vaccines, there has been clear evidence of the strong protection the vaccine offers them as well as other age groups. Overall, the CDC estimates that those who are unvaccinated are 6 times more likely to test positive for COVID-19, and over 11 times more likely to die from it.
Although age has been the greatest predictor for severe illness, as more adults have gotten vaccinated, the proportion of cases in children has risen, shifting focus to the vaccination of that group.
Recently, Pfizer and BioNTech announced positive results for their clinical study in children ages 5 to 11. An FDA advisory committee recommended it for emergency use authorization in that age group, and the FDA then granted a smaller dose of Pfizer’s COVID-19 vaccine for emergency use. The CDC has also recommended the low-dose shots, and the rollout for the 28 million U.S. children in this age group is underway.
California Governor Gavin Newsom announced plans to make COVID-19 vaccines mandatory for in-person school attendance from kindergarten to 12th grade following full FDA approval for a student’s age group. Full approval could follow the emergency use authorization by several months.
To help answer the questions many parents have, we reached out to two experts from UC Davis Health, Dean Blumberg, chief of pediatric infectious diseases at UC Davis Children’s Hospital, and Lorena Garcia, professor of epidemiology at UC Davis School of Medicine.

Dean Blumberg, chief of pediatric infectious diseases at UC Davis Children’s Hospital

Lorena Garcia, professor of epidemiology, UC Davis School of Medicine

What are the current rates of cases/hospitalization/death in children 5-11 years old?
Lorena Garcia: To date, 1.9 million COVID-19 cases have been reported in children 5-11 years old (roughly 7% of that population). During the large wave last winter, children in this range had a lower rate of infection compared to adults, however in the recent wave starting last July the rate increased and has remained closer to that of young adults. Hospitalizations in this age range have been the lowest of any age group but have also increased over time (see chart below), reaching 24 per 100,000 in August of 2021. 160 deaths have been reported in this age group to date, a rate of 0.008% deaths per case. For comparison, 112 children ages 5-17 died due to influenza in the 2019-20 flu season. That number was slightly higher (156) in the 2018-19 flu season.
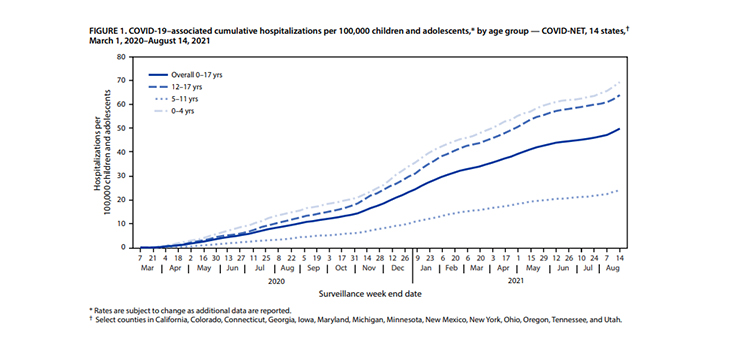
Are there factors that increase susceptibility and the risk of hospitalization and death for children in this age range?
Dean Blumberg: Children with underlying medical conditions are at increased risk for more serious disease. This can include heart, lung or kidney diseases, weakened immune systems, or chronic illnesses such as obesity or asthma. In fact, two-thirds of children hospitalized with COVID-19 have at least one underlying condition. And children with obesity are 30% more likely to be hospitalized with COVID-19 compared to children without obesity.
Lorena Garcia: Socioeconomic factors including poverty, unequal access to health care, poor environmental conditions and educational inequities increase the risk of infection, severe illness and death in children in this age range. These often translate directly into a disparate impact on minority communities.
When normalized for population distribution, Black, Hispanic and American Indian and Alaska Native children have the highest rate of hospitalization and death – 2 to 4 times those observed for White and Asian children. Children of Asian descent had the lowest rate of infection, hospitalization and death.A key intermediary for the disparate impact in these communities may be the (2-3x) higher rates of existing medical conditions described above by Dr. Blumberg. Differences in vaccination rates also contribute to the trends, with Asian children having the highest vaccination rates and Black children having the lowest.
What about MIS-C, the more serious illness that some children experience with COVID-19?
Dean Blumberg: Multisystem inflammatory syndrome in children (MIS-C) occurs 2-4 weeks following acute COVID-19 infection and involves inflammation of several different organ systems. It may involve the heart, lungs, kidneys, brain, skin, eyes, or gastrointestinal organs. MIS-C can be serious, even deadly (1-2% die), with the vast majority of children hospitalized and more than half admitted to intensive care. Fortunately, most children who were diagnosed with this condition have gotten better with specific medical care. This occurs in approximately one out of every 3,000 children (up to 21 years of age) who get COVID-19. The most common age for MIS-C is 5-11 years of age. Racial and ethnic minorities are at increased risk for MIS-C, with over 60% of cases occurring in Hispanic/Latino or Black non-Hispanic children.
Do children get “long COVID”?
Dean Blumberg: Long COVID occurs when symptoms linger four or more weeks after an acute infection. There are a wide range of health problems associated with long COVID including headache, “fuzzy thinking”, fatigue, sleep difficulties, headaches, loss of taste and smell. It occurs in children, but appears to occur about 50% less commonly compared to adults. A recent study found 7-8% of children with COVID-19 reported continued symptoms more than 12 weeks after diagnosis. The impact on quality of life during this period may be significant with limitations of physical activity, feeling distressed about symptoms and mental health challenges. These may all result in decreased school attendance and participation.
Are transmission rates different in children of this age range? Do we know if the vaccine reduces transmission rates?
Dean Blumberg: Some studies suggested that infection and transmission rates were lower in children compared to adults, but others suggest they are similar. Several factors including variants and vaccines may explain the different results. What we know with more clarity is that vaccination reduces the risk of infection, which in turn decreases the risk of transmission to others, including family members.
What have we learned about the spread of COVID-19 in schools?
Lorena Garcia: The Centers for Disease Control and Prevention has provided guidance based on current scientific evidence and lessons learned from schools. The evidence shows that implementing layered prevention strategies can reduce the spread of COVID-19 to levels below community settings. These include wearing masks, washing hands, physical distancing, cleaning and disinfecting, ventilation, as well as testing, tracing and quarantining programs.
Is the recently approved vaccine for children the same or different from the version for adults?
Dean Blumberg: The dose of the Pfizer/BioNTech vaccine is one-third the dose compared to older children and adults. It is not uncommon for pediatric vaccine doses to be lower than doses for older people since the immune response is robust in younger individuals.
The mRNA used in the vaccine is the same, but the use of buffering agents differ slightly to help maintain the pH and stability for extended storage periods.
Can you provide insight about the clinical trial conducted for this age group?
Dean Blumberg: 2,268 children 5-11 years of age were included in the pivotal study evaluated by the FDA for the emergency use authorization. That sample size is similar to the study used for 12–15-year-olds. 1,518 children received two doses of vaccine 21 days apart, while 750 children received a placebo. There were three symptomatic COVID-19 cases in the vaccine group and 16 in the placebo group, resulting in 91% effectiveness.
The antibody responses after vaccination were the same compared to older children and young adults. The side effects after vaccination were also similar, although the younger children tended to have milder side effects. For example, pain at the injection site was twice as common after vaccination compared to the placebo, but severe pain was uncommon.
Are there any groups in this age range that are at higher risk of adverse events from the vaccine? Why did Sweden and Denmark pause the rollout in children?
Dean Blumberg: Anyone with a severe allergic reaction to a previous dose of COVID-19 vaccine or any of the vaccine components should not be vaccinated. Some Scandinavian countries suspended Moderna vaccine use in children and young adults (under30 years of age) due to concerns related to myocarditis or heart inflammation that can occur, rarely, after vaccination. The CDC and others have studied this in the US and concluded that it is far safer for children to be vaccinated compared to being vulnerable to COVID-19 infection which may also cause myocarditis.
Other vaccines are already required for schools. How is this vaccine similar or different?
Lorena Garcia: Vaccines have been one of the most effective tools in reducing the risk of vaccine-preventable childhood diseases. Requiring a vaccine for students is not new. With the addition of COVID-19, California now requires ten immunizations. The Pfizer/BioNTech vaccine uses a new approach called mRNA, but offers a similar rate of effectiveness as the polio (99%), measles (97%) chickenpox (90%) and mumps (88%) vaccines. Each disease and related vaccine has its own risk/benefit profile. In terms of transmission, COVID-19 is less transmissible (3-7 transmissions per infection) than measles (12-18 transmissions) and chickenpox (10 transmissions), but similar to polio (4-6 transmissions). The case mortality rate for COVID-19 (0.008%) is less than that for measles (0.1%) and polio (0.05 – 0.025%), but higher than chickenpox (0.003%).
What can be done to address disparities in health and economic impact for children in this age range?
Lorena Garcia: As described above, the health and economic toll can have a larger effect in under resourced communities. In order to help mitigate the disparities, we need to ensure proportionate access to care and the vaccine, flexibility for time off to receive the vaccine and replacement of wages when sick or caring for a family member that is sick. For immigrants, we also need to address language barriers and fear of deportation. Community centered mitigation policies should include widely accessible integrated healthcare delivery, transportation to medical appointments, language services, community health workers, long-term community investment (e.g. affordable housing, jobs), and systematic collection of data, including race and ethnicity and the social determinants of health such as housing, food, transportation, utilities, child care, employment, education and finances. We also need mechanisms and community partnerships to deliver scientifically sound information regarding vaccines and diseases.
Additional Resources
Vaccines and children webinar
FAQ about the COVID-19 vaccine and kids







