UC Davis licenses novel compound that helps stem cells regenerate bone to treat bone diseases
Hybrid molecule LLP2A-Alendronate could have implications for osteonecrosis, fractures, osteoporosis and inflammatory arthritis.
From Left: Fred Tileston (RABOME, Inc.), Ruiwu Liu, Nancy Lane, Christy Pifer, Wei Yao, Kit Lam and Jiwei Chen (RABOME, Inc.)
The University of California, Davis, is pleased to announce a licensing agreement with Regenerative Arthritis and Bone Medicine, Inc. (RABOME) for a class of drugs developed at UC Davis that hold potential for treating diseases associated with bone loss and inflammatory arthritis.
The license, negotiated by the InnovationAccess team within the UC Davis Office of Research, provides the university affiliated startup with rights to four families of patents and patent applications related to the novel composition of a hybrid molecule, LLP2A-Alendronate, which has been found to effectively direct mesenchymal stem cells (MSCs) to induce bone regeneration in animal models. The compound works by guiding transplanted and endogenous MSCs to the surface of the bone where they differentiate into bone-forming cells, thereby increasing bone mass and strength. These cells are also immune-modulating which help to reduce inflammation at the target sites.
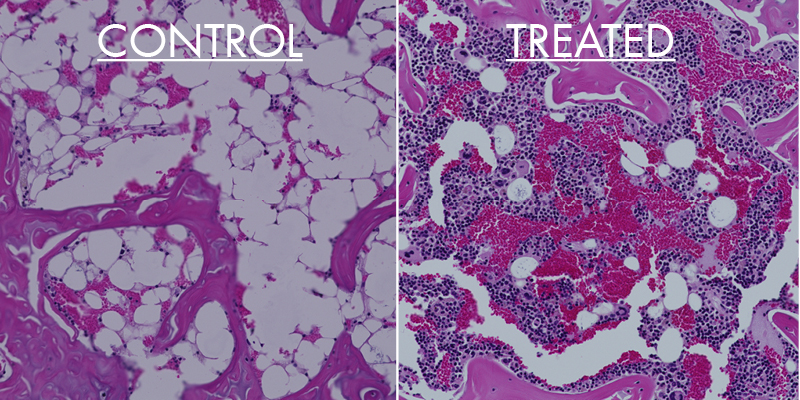
Distal femur from mouse showing more bone marrow (pink) and bone marrow filled with red sinusoids, a sign of higher vascularity, when treated with LLP2A-ALE for 90 days.
The use of stem cells as therapeutic agents is a growing field, but directing stem cells to travel and adhere to the surface of bone for bone formation has been an elusive goal in regenerative medicine.
“There are many stem cells, even in elderly people, but they do not readily migrate to bone,” said Wei Yao, co-inventor and associate professor at UC Davis. “Finding a molecule that attaches to stem cells and guides them to the targets we need provides a real breakthrough.”
Translating discovery into societal and commercial impact
Late last year, RABOME received approval from the Food and Drug Administration to begin Phase I clinical trials to evaluate the safety of the drug in humans. The study sites are currently screening patients for enrollment.
“We are pursuing several indications for use, but our initial focus is in developing a treatment for osteonecrosis, a disease caused by reduced blood flow to bones,” said Fred Tileston, president and chief executive officer of RABOME. As many as 20,000 people per year in the United States develop osteonecrosis.
RABOME also plans to pursue other indications for use including fracture healing, osteoporosis and inflammatory arthritis.
“We are pleased that this very promising technology is being shepherded by Mr. Tileston, who is an experienced business leader and entrepreneur,” said Dushyant Pathak, associate vice chancellor for Technology Management & Corporate Relations at UC Davis. “It is exciting to see the team’s progress in translating the discovery into commercial and societal impact.”
Breaking barriers through cross-discipline collaboration
The development of the novel therapy is the result of a successful research collaboration between two teams at UC Davis: a group of experts on bone health, led by Nancy Lane and Wei Yao from the Center for Musculoskeletal Health, and a group of medicinal chemists led by Kit Lam and Ruiwu Liu from the Department of Biochemistry and Molecular Medicine.
“This research was a collaboration of stem cell biologists, biochemists, translational scientists, a bone biologist and clinicians,” said Lane. “It was a truly fruitful team effort with remarkable results.”
Lane and Yao received a Disease Team Therapy Development research grant in 2013 from the California Institute for Regenerative Medicine (CIRM), which along with federal grants from the NIH, supported the preclinical research. CIRM was established in 2004 via California Proposition 71 to fund stem cell research in attempt to accelerate and improve treatments for patients where current needs are unmet.
About RABOME, Inc.
RABOME, Inc., was launched in 2013 to commercialize a class of drugs based on targeting mesenchymal stem cells to various sites of clinical relevance. The company is located in Hillsborough, California.
Media Links
- Stem cell agency commits $150 million to develop new therapies
- UC Davis investigators develop method of directing stem cells to increase bone formation and bone strength
- UC Davis InnovationAccess
Media Contact
- AJ Cheline, UC Davis Office of Research, 530-752-1101, [email protected]





