IACUC – Frequently Asked Questions (FAQS)
- What is the purpose of the Animal Care and Use Program?
- How do I dispose of a Controlled Substance?
- What species are covered by the USDA Animal Welfare Act?
- Where can I access the Occupational Health Program Application and what are the steps?
- How often do I need to take the Animal Care and Use 101 class?
- Are laboratory animal skills courses available?
- When do I need to submit an Animal Care and Use protocol?
- How can I access an Animal Care and Use protocol online?
- How do I submit an Animal Care and Use protocol?
- How do I amend an Animal Care and Use protocol?
- What could happen if I perform procedures that are not covered by my Animal Care and Use Protocol?
- Can I house animals in the laboratory overnight?
- What is the review process for Animal Care and Use Protocols and amendments?
- Who needs to be listed on an Animal Care and Use Protocol?
- What are the minimum requirements for staff to be listed on an Animal Care and Use Protocol?
- Who can complete the “Risk Assessment” portion of the Occupation Health Program when adding new staff to protocol rosters?
- When can I submit an amendment instead of writing a new Animal Care and Use Protocol?
- Who is eligible to be a PI on an Animal Care and Use Protocol?
- How can I add or remove personnel from an Animal Care and Use Protocol?
- How can I verify that I have been added to an Animal Care and Use Protocol?
- What is the typical Animal Care and Use Protocol turn-around time? What about for amendments?
- What forms need to be completed in addition to the Animal Care and Use Protocol form?
- Do I need Biological Use Authorization (BUA) before my Animal Care and Use Protocol can be approved?
- Do I need Radiation Use Authorization (RUA) before my Animal Care and Use Protocol can be approved?
- Do I need authorization to include the use of stem cells in my Animal Care and Use Protocol?
- Do I need an Animal Care and Use Protocol if I will only be observing the animals in their natural environment (without manipulating their environment)?
- In my Animal Care and Use Protocol, do I have to include the breeding animals, or just experimental animals?
- What is a power calculation and are there power calculation programs available?
- How long is my Animal Care and Use Protocol approved for?
- I will be collecting wild animals for my work at UC Davis. Do I need other authorizations besides one from the IACUC in the form of an Animal Care and Use Protocol?
- Why is a literature search required when writing an Animal Care and Use Protocol?
- How can I obtain a letter of verification for a funding agency?
- I need the UC Davis Animal Welfare Assurance number for my grant application. What is that number?
- What is the UC Davis USDA Registration number?
- What is the UC Davis AAALAC Accreditation status?
What is the purpose of the Animal Care and Use Program?
Please see the IACUC Charge
How do I dispose of a Controlled Substance?
Please use the online Controlled Substance Usage Log Management System to submit a disposal request. Only authorized custodians, contacts, and users can submit disposal requests. Once received, you will be contacted by an Office of Environmental Health and Safety (EH&S) staff member to set an appointment for the substance(s) to be officially transferred to an EH&S representative. https://safetyapps.ucdavis.edu/EHS/CSubstance/usageLog/index.cfm
What species are covered by the USDA Animal Welfare Act?
The AWA defines a covered animal as:
…any live or dead dog, cat, nonhuman primate, guinea pig, hamster, rabbit, or any other warm-blooded animal, which is being used, or is intended for use for research, teaching, testing, experimentation, or exhibition purposes, or as a pet. This term excludes birds*, rats of the genus Rattus, and mice of the genus Mus, bred for use in research; horses not used for research purposes; and other farm animals, such as, but not limited to, livestock or poultry used or intended for use as food or fiber, or livestock or poultry used or intended for use for improving animal nutrition, breeding, management, or production efficiency, or for improving the quality of food or fiber. With respect to a dog, the term means all dogs, including those used for hunting, security, or breeding purposes.
*Birds (other than those bred for research) are covered under the AWA. New regulatory standards were published on February 21, 2023 (https://www.aphis.usda.gov/aphis/ourfocus/animalwelfare/new-bird-rule/awa-standards-for-birds)
Where can I access the Occupational Health Program Application and what are the steps?
Please visit the Occupational Health Surveillance System Website to access the application.
The OHSS process consists of multiple steps; often people will do steps 1-3 and think they are finished.
The OHSS process is described below:
- The PI/supervisor creates a Risk Assessment (RA) (or requests renewal of existing assessment) for the participant online at https://ehs.ucop.edu/ohss. Sometimes the PI and participant are the same person; in this case both the participant and the PI steps will need to be completed by them.
- The participant reviews the RA and either accepts it or rejects it if changes are needed.
- After accepting the RA, the participant fills out the confidential Health Questionnaire (HQ) and submits it for review by a Health Professional. (Make sure the participant has their vaccination history available as vaccine dates are requested on the HQ.) Do not send personal medical information to IACUC staff or your supervisor.
- The UC Davis Occupational Health medical professional reviews the participant’s Risk Assessment and Health Questionnaire and sends the participant an email with a link to their Medical Assessment.
- The participant logs on to the OHSS site and reviews/acknowledges the Medical Assessment. If there are questions from the medical professional on either the Health Questionnaire or Risk Assessment or recommendations requiring follow-up, the participant will need to respond to the questions or contact Occupational Health Services for an appointment at 530-752-6051 to complete the process.
- The IACUC system will be updated once steps 1-5 are complete and required in person appointments or procedures have been cleared. The review process may take several working days. In some cases, consultation with a UC Davis Occupational Health Services medical professional or medical services (i.e., TB testing, respirator clearance, vaccinations) may be required prior to obtaining clearance to enter certain facilities. Either the participant or the person who filled out the Risk Assessment may make the appointments required for testing. A Request for Service document may need to be sent before the appointment can be made with Occupational Health Services.
- Please note that the OHSS system updates the IACUC database overnight so if someone is cleared in the OHSS system today, it will not be possible to add them to a protocol until tomorrow.
How often do I need to take the Animal Care and Use 101 class?
Animal Care and Use 101 training is to be renewed every three years. If you are listed on an active Animal Care and Use Protocol staff roster, prior to the 3-year anniversary of your training date, you will receive an email prompting you to update your training on-line. You have the option to take the Animal Care and Use 101 Retraining Quiz online or retake the Animal Care and Use 101 online course.
Are laboratory animal skills courses available?
The IACUC office offers a variety of courses that pertain to laboratory animal care and experimentation (handling, restraint, injections, surgical skills and blood collection). Additional animal training is available upon request. For further information, please visit the Training Resources page here.
UC Davis is subscribed to the American Association for Laboratory Animal Science (AALAS) Learning Library. The AALAS library offers a wide variety of online training courses. Courses include training for the AALAS certification exams (ALAT, LAT, LATG), training on techniques for working with a variety of species, and regulatory training. A complete list of the courses is available at the AALAS learning library website. We encourage you to take advantage of these training opportunities. If you would like access to these courses, please send an email to the IACUC Office at [email protected] to request a login ID and password.
When do I need to submit an Animal Care and Use protocol?
An Animal Care and Use Protocol must be submitted when university owned animals are used for research, teaching and training. Please see the IACUC policy on information regarding trials in non-university (client) owned animals.
How can I access an Animal Care and Use protocol online?
To view a protocol please use the Online Protocol and Amendment System and choose “View This Active Protocol”. You will need a UC Davis Kerberos login and password to enter this system.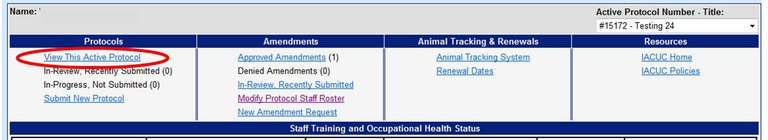
How do I submit an Animal Care and Use protocol?
To submit a protocol please use the Online Protocol and Amendment System and choose “Submit New Protocol”. You will need a UC Davis Kerberos login and password to enter this system.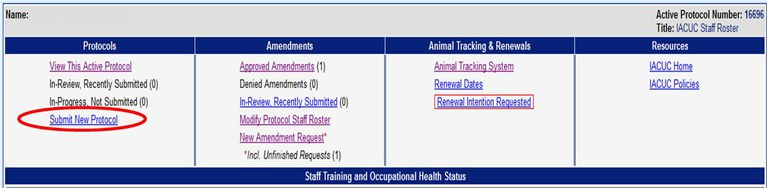
How do I amend an Animal Care and Use protocol?
To submit an amendment please use the Online Protocol and Amendment System and choose “New Amendment Request”. You will need a UC Davis Kerberos login and password to enter this system.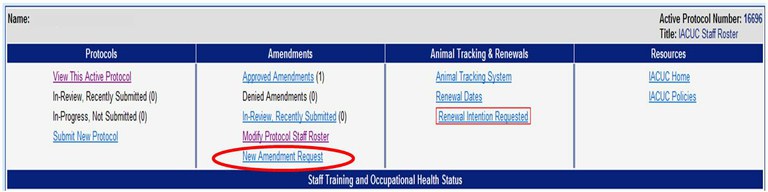
What could happen if I perform procedures that are not covered by my Animal Care and Use Protocol?
The IACUC will review the Animal Care and Use Protocol deviation and may send a letter to the Principal Investigator to communicate the importance of making sure that the actual work matches what is described in the Animal Care and Use Protocol. The IACUC may also choose to suspend personnel or the entire Animal Care and Use Protocol (please see the IACUC Policy on Protocol Suspension. Depending on the severity, the IACUC may be required to send a letter to the Office of Laboratory Animal Welfare (OLAW) and also to the funding agency regarding the Animal Care and Use Protocol deviation, and the funding agency may choose to withhold funding for the period of non-compliance. To prevent these from happening, please make sure that you do not perform procedures that are not already approved by the IACUC. You can submit an amendment to your Animal Care and Use Protocol using the IACUC Online Protocol and Amendment System. You can also read OLAW’s Guidance on this topic at https://olaw.nih.gov/faqs#/guidance/faqs?anchor=50307 and http://grants.nih.gov/grants/guide/notice-files/NOT-OD-05-034.html.
Can I house animals in the laboratory overnight?
Research animals must be housed in approved, dedicated animal housing facilities (vivaria); they may not be housed/held in a PI’s laboratory overnight unless scientifically justified in an IACUC approved protocol or amendment. If animals are to remain in a non-vivaria space for more than 12 hours, the Principal Investigator (PI) must obtain IACUC approval and include Standard Operating Procedures (SOP) forms for husbandry procedures while housed in the laboratory. Please see IACUC policy 19 Laboratory Housing for Research Animals and the template for the required Standard Operating Procedures (SOP) forms for housing animals in the laboratory and an example of a filled out SOP.
What is the review process for Animal Care and Use Protocols and amendments?
Please see IACUC Policy 7 Animal Care and Use Protocol/Amendment Review and Renewal Process
Who needs to be listed on an Animal Care and Use Protocol?
Per UC PPM 29-30 “All personnel working on teaching or research projects involving live vertebrate animals must be listed on the protocol roster (not required for unpaid students in the laboratory for under one quarter or students in the classroom)” Please note that in order to be placed on an Animal Care and Use Protocol, staff must complete Animal Care and Use 101 and be enrolled in the Occupational Health Program.
What are the minimum requirements for staff to be listed on an Animal Care and Use Protocol?
Before personnel can be added to an Animal Care and Use Protocol they must first participate in the Occupational Health Program. IACUC policy 25 Animal Care and Use Occupational Health Program explains how to enroll an individual in the program with alternate options for students in classes, volunteers, and Temporary Affiliates. They must also complete the Animal Care and Use 101 online course. Any person(s) being listed as a surgeon for rodent survival surgeries, must complete the online Rodent Survival Surgery course. For further information about training requirements please refer to “IACUC Policy 2 “Training Requirements for Personnel Working with Live, Vertebrate Animals Use in Research and Teaching””
Who can complete the “Risk Assessment” portion of the Occupation Health Program when adding new staff to protocol rosters?
In addition to the Principal Investigator (PI), the alternate contact, lab manager or supervisor can complete the Occupational Health, Risk Assessment form. Please note that renewal reminders are sent from the OHSS to the person who submitted the Risk Assessment and the individual.
When can I submit an amendment instead of writing a new Animal Care and Use Protocol?
An Animal Care and Use Protocol can be amended to include changes only if the changes are consistent with the original protocol objectives. If justification cannot be provided as to how the proposed changes fit in with the original protocol, a new Animal Care and Use Protocol should be submitted. Examples of acceptable amendments include:
- Change in protocol title, PI, alternate contact or funding source
- Addition or change in the location of animal use
- Request for additional animals
- Additional strains or species requests
- Modifications in drug dose and route of administration
- Modification or addition of procedures (experimental, surgical, housing)
- Changes in anesthetics or analgesics
- Changes to methods of euthanasia
- Modifications in pre-, intra-, and post-operative care
Who is eligible to be a PI on an Animal Care and Use Protocol?
The Principal investigator (PI) must be a University of California faculty member or employee with career status. Non-university of California personnel, graduate students and residents may be the alternate contact on an Animal Care and Use Protocol.
How can I add or remove personnel from an Animal Care and Use Protocol?
Please use the Online Protocol and Amendment System and choose “Modify Protocol Staff Roster”. You will need a UC Davis Kerberos login and password to enter this system. Please do not submit staff roster changes by using the “New Amendment Request” tab unless a change in the Principal Investigator or Alternate Contact is being requested.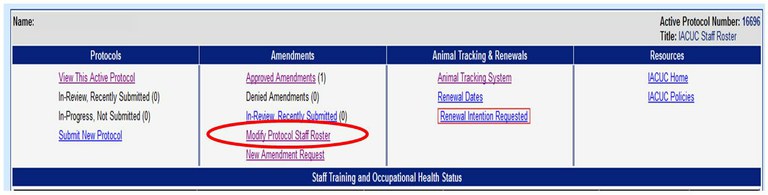
To add personnel choose “Add Personnel”.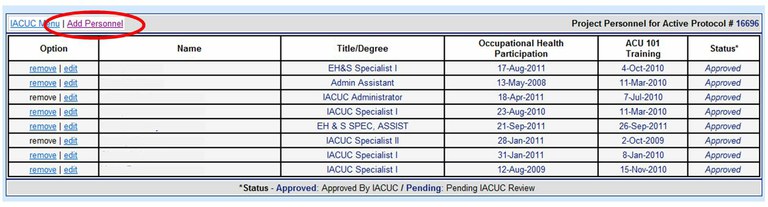
To remove personnel choose “remove”.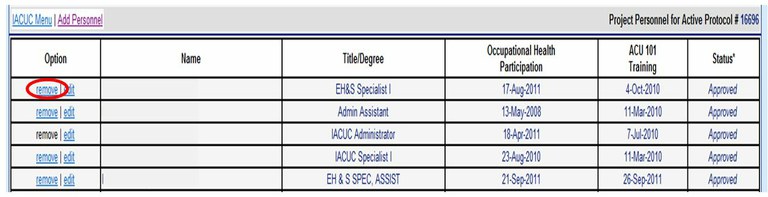
How can I verify that I have been added to an Animal Care and Use Protocol?
To verify that you have been added to a protocol, you can view the protocol staff roster (section 18 of protocol) by opeing the protocol using the Online Protocol and Amendment System and choose “View This Active Protocol”. You will need a UC Davis Kerberos login and password to enter this system. Please note that if you are unable to access the protocol then you have not been added to it. Please consult with your PI and ensure that you have met the requirements to be added. For information on what is required to be on a protocol please see the FAQ- What are the minimum requirements for staff to be listed on an Animal Care and Use Protocol?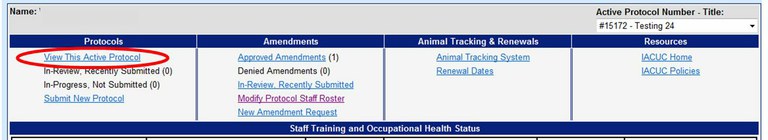
What is the typical Animal Care and Use Protocol turn-around time? What about for amendments?
Typically, from the date of submission, the average turn-around time for a protocol is approximately 6 weeks for protocols and approximately 4 weeks for amendments. Please note that this is an average and more complicated protocols (with surgery, hazards, multiple procedures, etc.) will require longer for review. The review process has multiple steps that must occur prior to an item being placed on an agenda, therefore early submission is highly recommended to avoid delays in your research and teaching. For more information on the review process please refer to “IACUC Policy 7 “Protocol and Amendment Review Process”.
What forms need to be completed in addition to the Animal Care and Use Protocol form?
Under the following circumstance the indicated form is required.
- If hazardous chemicals, biological agents, or radioisotopes are listed on the protocol, they must be included in section 8 and 14 of the protocol. The protocol will be reviewed by EH&S at the time of IACUC review and a Vivarium Hazard Safety Sheet may be attached.
- If you have been approved to house animals in the laboratory for more than 12 hours then a Standard Operating Procedure (SOP) form must be completed indicating how the animals will be cared for while being housed in the laboratory.
Do I need Biological Use Authorization (BUA) before my Animal Care and Use Protocol can be approved?
If proposed Animal Care and Use Protocols include the use of recombinant DNA, human tissues, human cells, stem cells, viral vectors, generation of knockout or transgenic mice, or any infectious agent, BUA is required before the Animal Care and Use Protocol can be approved. For BUA instructions or any questions regarding BUA requirements, please contact the Biological Safety Office.
Do I need Radiation Use Authorization (RUA) before my Animal Care and Use Protocol can be approved?
If proposed Animal Care and Use Protocols include the use of radioactive material, RUA is required before a Animal Care and Use Protocol can be approved or any questions regarding RUA requirements, please contact the Radiological Safety Program or Environmental Health and Safety Office at 530-752-1493.
Do I need authorization to include the use of stem cells in my Animal Care and Use Protocol?
Any questions pertaining to stem cell work should be directed to the UC Davis Stem Cell Research Oversight (SCRO) Committee for approval.
Do I need an Animal Care and Use Protocol if I will only be observing the animals in their natural environment (without manipulating their environment)?
No. UC Davis Animal Care and Use Protocols are only needed when you will be working with live vertebrate animals in a teaching and/or research activity on behalf of UC Davis.
In my Animal Care and Use Protocol, do I have to include the breeding animals, or just experimental animals?
Animal numbers are determined by the number of breeders and the TOTAL estimate of the number of pups generated. If only a percentage of the pups can be used for your project (e.g., only male pups, a specific genotype, etc.), that estimate should be included in the calculation of the final number required for the study. See IACUC Policy 20 “Numbers Rationale for Animals in Protocols”
What is a power calculation and are there power calculation programs available?
Power calculations are statistical analyses that can be done prior and after collecting data. Power analysis that is done before data collection is typically used to estimate the sample size needed to achieve power (statistical significance). Power analysis can be used to determine the approximate minimum number of animals required so that an effect can be detected within the given sample size. Power calculations can also be used to calculate the minimum effect size that is likely to be detected in an experiment with a given sample size. For more information regarding power analysis please see IACUC Policy 20 “Numbers Rationale for Animals In Protocols” for further information and examples.
How long is my Animal Care and Use Protocol approved for?
Protocols must be renewed annually (not re-written) but three years after the initial date of IACUC approval, a protocol will expire and cannot be renewed through the annual renew process. A new protocol request must be submitted prior to the three year expiration date to keep the study active.
I will be collecting wild animals for my work at UC Davis. Do I need other authorizations besides one from the IACUC in the form of an Animal Care and Use Protocol?
This will be determined by the agencies involved. Please check with US Fish and Wildlife Service, California Department of Fish and Wildlife, and/or any other agency which may govern work proposed in your Animal Care and Use Protocol.
Why is a literature search required when writing an Animal Care and Use Protocol?
The Animal Welfare Act (AWA) regulations require principal investigators (PIs)/scientists to consider alternatives to procedures that may cause more than momentary or slight pain or distress to the animals. They must provide a written narrative to their Institutional Animal Care and Use Committee (IACUC) that describes the methods and sources used to determine that alternatives were not available (9 C.F.R. § 2.31 (d)(ii)(2022)). Additionally, PIs/scientists must also provide written assurance that their activities do not unnecessarily duplicate previous experiments (9 C.F.R. § 2.31 (d)(iii)(2022)).
Alternatives to be considered include those that would:
- Replace animals with non-animal alternatives
- Refine the procedures to minimize discomfort that the animal(s) may experience
- Reduce the number of animals used overall
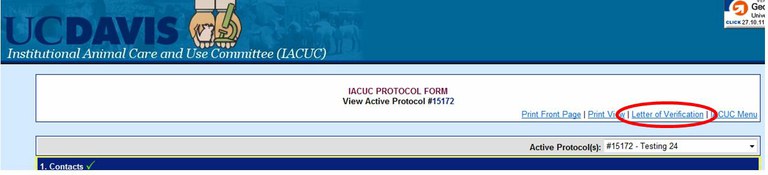
How can I obtain a letter of verification for a funding agency?
Use the Online Protocol and Amendment System and choose the protocol you would like the letter of verification for. You will need a UC Davis Kerberos login and password to enter this system.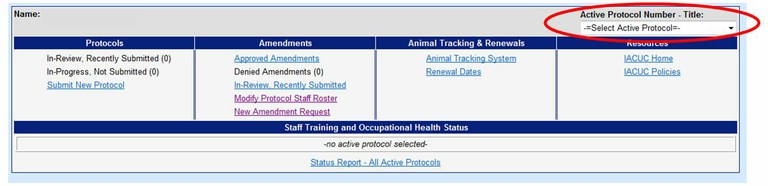
Choose “View This Active Protocol”

Choose the “Letter of Verification” tab at the top right corner of the online protocol form. This should open the Letter of Verification in a new window.
I need the UC Davis Animal Welfare Assurance number for my grant application. What is that number?
Grant applications often ask for a “PHS Animal Welfare Assurance Number”. There is a single Assurance Number for the campus: A3433-01.
What is the UC Davis USDA Registration number?
The UC Davis USDA Registration number is: 93-R-0433.
What is the UC Davis AAALAC Accreditation status?
The UC Davis Animal Care and Use Program is Accredited by AAALAC International. Our AAALAC number is 000029. We have been accredited since 1966 and our most recent accreditation letter date is: July 7, 2023.

